
Empower Your
Regulatory Teams
Simplify compliance by providing your teams with the tools to maintain accuracy, consistency, and efficiency while minimizing recall risks throughout the process.
5000+ Brands











Collaborate better and minimize delays



Artwork Compliance Check
Ensure precise compliance checks by utilizing proofing tools to validate pack information, font, and format consistency. Stay on track with comprehensive checklists to verify all required compliance details, eliminating the risk of costly errors down the line.
Audit Trail
Maintain complete records of changes with timestamps and user details. This essential feature facilitates seamless regulatory audits, ensuring compliance and transparency for all stakeholders.
Track and Manage Regulatory Dates
Effortlessly monitor key regulatory dates with our reliable system. Stay on schedule, track approvals, and ensure compliance across the entire artwork lifecycle.
Ensure error-free artwork with AI-powered proofing tools designed for regulatory accuracy
Manage Compliance Needs Across Markets
Efficiently track and manage market-specific compliance requirements from a unified platform. Streamline the process of ensuring packaging meets regulatory standards globally while reducing the complexity of handling different market rules.


Maintain Error-Free Packaging
Automate the packaging review process to eliminate human errors. Ensure compliance by checking key elements like text, font, and allergens, enhancing label accuracy and delivering consistent, high-quality packaging.
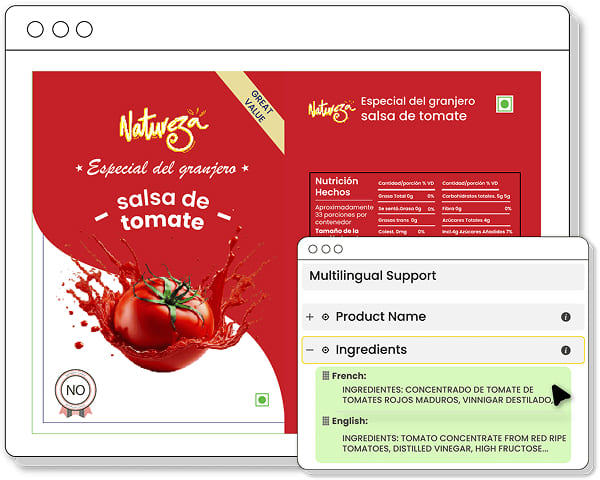
Simplify Multilingual Packaging Management
Manage multilingual packaging effortlessly by ensuring accurate translations, consistent terminology, and correct formatting for all language versions, helping streamline the adaptation process across various regions without errors.

Enhance Team Collaboration for Regulatory Compliance
Facilitate smooth communication and collaboration across teams by centralizing information and enabling real-time updates, ensuring timely delivery of compliant packaging and minimizing delays in the approval process.
Things You Might Be Wondering About
Does the system alert us to upcoming regulatory deadlines?
Yes, you can set regulatory required dates with automated reminders and escalation paths.
Can I audit who changed regulatory content and when?
Yes, every change is logged with username, time, and previous versions retained.
How is version control maintained for regulatory audits?
Each version is archived with full traceability for regulatory inspections and audit reviews.
Can we run automated compliance checks on packaging?
Yes, built-in proofing tools validate content, font size, alignment, and formatting for regulatory accuracy.
Can we monitor packaging approvals by region or market?
Yes, a unified dashboard tracks requirements and approval statuses for every market or region.
Can we customize checklists for regulatory reviews?
Yes, configurable checklists help ensure all regulatory and market-specific requirements are verified before approval.
Still have questions?
Can’t find the answer you’re looking for? Please chat to our friendly team.





